Science on Tap: Introduction to the Science of Beer Aging
Aging a beer is a test of patience. You planned and brewed the perfect beer for aging, but how do you know if the flavors will become more rounded or if they will become dull and muddy? Twelve months later, your big spiced chocolate apricot stout is out on your table; your bottle opener in hand. Anticipation builds as you reflect on the plethora of aldehydes, ketones, esters and lactones swimming around your glass. Who am I kidding, it was more along the lines of: “hot-damn, I forgot about this beer, let’s drink it.”
Chemically speaking, beer is a water-ethanol solution containing hundreds of different molecules. These molecules come from the grain, hops, water, and yeast that we brew with. The process of mashing, boiling, and fermenting introduces new compounds and changes others. Everyone should be familiar with the primary process of C6H12O6 (glucose) being converted to 2 C2H5OH (ethanol) + 2 CO2 (carbon dioxide). However, we will discuss what happens to your beer after fermentation. And, since we are not making a neutral spirit, we have a wide variety of molecules and processes to contend with.

The components of freshly bottled beer are not in balance. Instead, they are in a dynamic state of change, under the influence of their environment. Consequently, molecules are subjected to many reactions during storage, the product of which determine the sensory characteristics of the beer. Anyone who has found an old, dusty homebrew or participated in a vertical tasting of the same beer over many years can appreciate how flavors can change, for better or worse. Since homebrewers don’t usually have access to the equipment used to measure the presence and abundance of hundreds of molecules in their beer (usually mass spectrometry (MS) or nuclear magnetic resonance (NMR)), we rely on our own sensory equipment (schnoz and kisser to be technical).
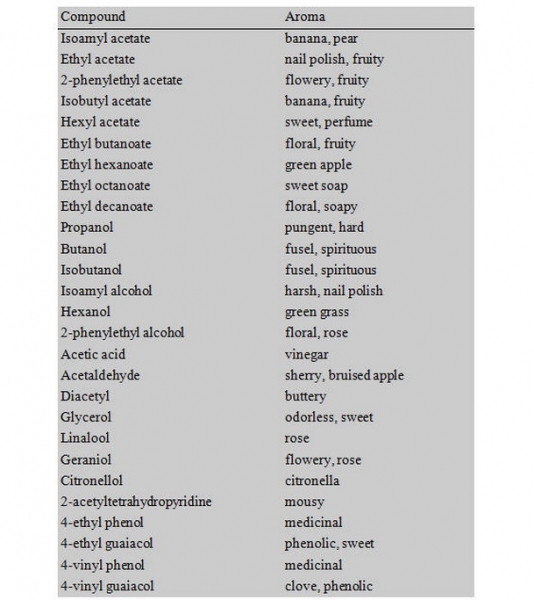
I emphasize the sensory aspect of aging in order to relate the molecular evaluation of beers to what most of us are tasting. An article describing the dynamics and influence over time of dimethyl trisulfide and gamma-nonalactone would be useful for some, but it is easier to relate to descriptions of the change in oniony and fruity/peachy flavors instead. For pallet training, I recommend a simple sensory test using your favorite BMC beer. This process can dramatically improve your recognition of flavors in beer and likely make you a better homebrewer. These products can usually be found at the grocery store or LHBS:
- Acetaldehyde: imitation apple flavoring (8 drops/12 oz. beer)
- Diacetyl: imitation butter flavor (4 drops/12 oz.)
- Acetic: vinegar (25 drops/12 oz.)
- Lactic: lactic acid (20 drops/12 oz.)
- Astringent: Tannin (? tsp into 1/4 cup water/6 pack) – this is a tricky one
- Phenolic: chloraseptic or sore throat spray (8 drops/12 oz.)
- Dimethyl sulfide: canned corn (1/4 cup strained liquid/12 oz.)
- Stale: small piece of cardboard in 12 oz.
- Meaty/Soy: soy sauce (5 drops/12 oz.)
At the very least, take two bottles from the same batch and store them at different temperatures for an advanced aging experiment. Put one bottle in the fridge for 10 days and hold the other bottle at 100F for 10 days (or as warm as you can get it).
Over time, particular compounds associated with particular flavors increase and decrease in a dynamic fashion, as different compounds form and degrade. For example, the compound (E)2-nonenal (an aldehyde), despite varying reports of its specific contribution, may be responsible for the cardboard flavor in stale beer. But is our beer actually stale because this compound is measured at a particular level? Not necessarily. We evaluate our beer based on taste. Of course, we are tasting the different contributions of these molecules, but the presence of off-flavor-associated molecules do not necessarily constitute an actual off-flavor in aged beer. This may be because we expect the beer to taste a certain way.
The formation and degradation of many compounds is possible over time. However, their relevance to beer aging is determined by the reaction rates under practical storage conditions (eg. temperature) and the taste of the brewer. This is where things become subjective. Different people prefer different flavors, and even beyond that, people perceive flavors differently. An objective measure of the abundance of molecular features may not coincide with the subjective taste of the brewer.
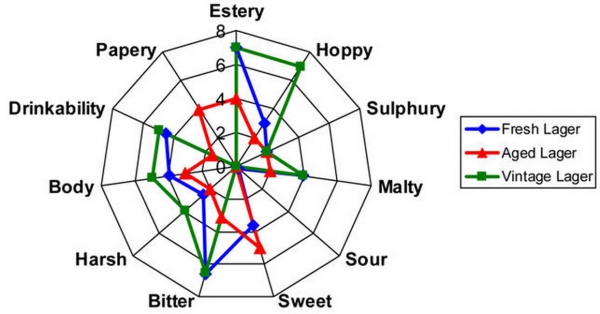
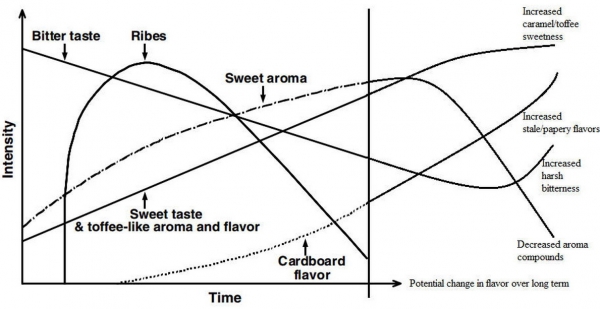
In 1977, C.E. Dalgliesh came up with a chart generalizing the flavor changes in beer over time. The ribes flavor and aroma is the aroma of Blackcurrant (Ribes nigrum), described as slightly fruity, but mostly like cat-piss, thanks to thiols (skunk and garlic aroma) and terpenes (why pine sap and hops smell so strongly). From this chart, we can see that bitterness and ribes declines over time while sweetness and staleness increases over time. However, since we are not tasting these components individually, we need to think about the contribution of all the flavors at a particular time. Taking all compounds into consideration, how will the flavor of the beer at time 1 be perceived compared to time 2? What are the flavor thresholds of the compounds that are responsible for the particular flavors?

Now, here you are thinking, “well, that depends…” Of course it does. Beer aging depends on many things. In terms of the environment, what can be controlled? Light exposure, temperature, and stability come to mind. The best place to age your beer is somewhere dark, cool, and still. If those variables are controlled, the next are process variables. The most important variable of this group is oxygen.
The impact of oxygen on long term storage will require its own article. In conjunction with temperature, oxidative processes will have the most impact on the flavor stability of your beer over time. The next major dependency is style. It is well known that particular styles age better than others. But what is it about the differences between these two groups that influences how they age? What features of a beer make it more amenable to aging?
Science on Tap – The Science of Beer Aging – Styles
We have all heard the rules for selecting a beer for aging: high ABV and low hops, dark-colored, imperial, etc. Really though, you can choose to age any beer. But why do some beers deteriorate deliciously while others degenerate disastrously? While the focus here is primarily post-fermentation, we can start with the components of particular styles that allow for better aging performance.
The storage conditions influence the speed and extent of the aging reactions that occur in beer, as mentioned before (Part 1). However, the concentration of the precursor chemicals involved in these reactions will also influence their progression. These precursors come from our choice of ingredients, and process variables (oxygenation, temperature, etc). Regardless of the particular style, a brewer must use well-practiced techniques with regard to sanitation, and choose fresh, high-quality ingredients. The best practice is to avoid generating off-flavor associated compounds from the start by removing chlorine/chloramine from your water, managing fermentation temperature, and avoiding oxygen and microbes. Rarely, does an off-flavor discovered in a fresh beer “age out” over time. However, this “aging out” can happen in certain instances, which I will discuss later.
Why do darkly colored beers age better than lightly colored beers? Generally, this has to do with the robustness and composition of the flavors. Darker beers are made from dark, roasted malts. These malts provide many flavorful volatile compounds. When brewing with these malts, we depend on a particular reaction to develop the sought after flavors: the Maillard reaction. The Maillard reaction is a non-enzymatic combination between an amino acid (from protein) and sugar (from mashing). Maillard reactions occur during malting and the boil, producing a huge diversity of products that contribute to flavor, and in particular, melanoidins.
Melanoidins form through a wide variety of chemical rearrangement and recombination of the Maillard products, resulting in darkly colored nitrogen based compounds that provide an intense malty character (some are also antioxidants and antimicrobials!). During aging, small products of the Maillard reaction can polymerize to form larger compounds with new flavors. In beers with a high percentage of dark malts, a higher proportion of particular flavor active compounds (carbonyls, Strecker aldehydes, furfural alcohols, and Maillard reaction intermediates), provide significant antioxidant capability. Together, many of these compounds are associated with staling in light colored beers, but in darkly colored beers, they provide a sweet and malty impression and enhance long-term flavor stability. The aging process of dark beers is characterized by the development of burnt, alcoholic, caramel, liquorice and astringent flavors. These strong flavors mask the evolution of stale flavors that is occurring concurrently. A word of caution though: The (often) higher acidity from roasted malts is hard on the yeast remaining in the beer during aging. This can result is meaty/soy sauce flavors as the yeast dies.
Why shouldn’t I age hoppy beers? You can, of course. But why would you? Even in the time it takes to finish a keg or case of bottles of your house IPA, most people can appreciate that the hop aroma (in particular) fades very quickly. The flavor eventually goes too. Moreover, a beer that is not highly hopped will be less likely to develop stale off-flavors. Bitter acids from hops (the iso-alpha, alpha, and beta acids) are quickly broken down, mostly due to oxidative processes. This degradation results in a decrease in bitterness (recall the Dalgliesh chart from part 1), but also in the formation of new compounds. These new compounds include a variety of carbonyls like (E)2-nonenal (stale), acetone (solventy), butanol and propanol (alcoholic/solventy), and butyric and propionic acid (cheesey/sulfur/sweaty). Again, I need to point out, that while these processes are likely occurring in an aged, hop-forward beer, it does not mean that these flavors are always detectable, nor considered off-flavors when the total flavor profile of the beer is considered. Regardless of the changes or flavors, you lose more than you gain when you age hoppy beers due to the abundance of precursor chemicals potentially resulting in off-flavors after degradation. A way to avoid some of these changes is to use hops with a high proportion of beta-acids (Lupulone, Colupulone, Adlupulone) like Amarillo or Galena. The beta-acids tend to maintain their bitterness over time and can age into fruity flavors as they degrade.
Why do high ABV beers age well? Alcohol is protective against age, in people and in beer. Generally, it is recommended that only beers >8% ABV be aged, and that beers >10% almost require aging. A high ABV beer is more resistant to contamination and spoilage by wild yeast or bacteria, thereby allowing the beer to sit for extended periods of time. Chemically speaking, ethanol in beer is an excellent scavenger of reactive oxygen species. Interestingly, so are residual sugars. A high ABV beer containing some dextrans will age better than its counterpart with reduced ABV and dextrans. As oxygen finds its way into your beer, either because you were not careful or just due to natural diffusion, it can react with metals (iron and copper from the water and malts) and form reactive oxygen species. Before aging, most high ABV beers are described as “hot” or “solventy” due to the high concentration of ethanol (and other alcohols such as fusels). Over time, the reactions between reactive oxygen and ethanol in beer results in the generation of flavorful fruity esters and a smoothing of the hot flavors. In some instances, depending on the oxygen content, an inappropriate conversion of ethanol to acetaldehyde then acetic acid can occur. Directly after fermentation, the presence and eventual disappearance of acetaldehyde is an example of when this “aging out” process can occur. Very fresh beer, or maybe better described as very new beer, can have an apparent apple flavor. The beer is sometimes described as “green” for two reasons: green as in young and inexperienced, and green as in green-apple flavored. Acetaldehyde is the chemical responsible for this flavor. During fermentation, yeast metabolizes sugar into ethanol with acetaldehyde as an intermediate. A high level of acetaldehyde in green beer is due to an incomplete fermentation. Allowing the beer to condition or age allows the yeast to complete their metabolism. However this process is different from the conversion of ethanol back to acetaldehyde, which can happen during extended aging.
Last but not least is yeast. Many of the aging process I have described are dependent on active yeast in the aging beer. Thus, filtered or pasteurized beer tends to not age as well as beer containing yeast. Primarily, you must choose a yeast that can handle the ABV of your beer and complete fermentation with the desired level of attenuation. Of equal importance, perhaps, is the flavor profile produced by the yeast during fermentation. Many long-aging beers are produced with ester-heavy yeasts. These (often fruity) esters produced during fermentation can undergo oxidation during the aging process and produce dried-fruit type flavors which complement the sweet, malty flavors in aged beer. For example, the ester isoamyl acetate (banana) produced during fermentation by many Belgian yeasts actually decreases over time in aged beer, while the esters gamma-hexalactone and gamma-nonalactone (peachy, fruity) increase over time.
Science on Tap: Temperature
Science on Tap: Effects of Temperature on Beer Aging
Over the course of the last two installments of Science on Tap, we have discussed, in general, the practice of long-term aging of beer (or cellaring), and why particular styles of beer are best suited for aging. There are many reactions occurring in your beer during aging, and storage temperature is the major driver of the rate for most of these reactions. Indeed, in commercial tasting panels, storage temperature had the greatest impact on flavor deterioration, haze, and color of the beer. The changes associated with high-temperature storage can occur quickly, and can be encountered often, at both the commercial scale, and the homebrew scale.
Storage temperatures are important to beer flavor. Beer contains a variety of compounds that are capable of autoxidation if temperatures get too high. This means that a beer that spent two weeks at 100F will have a similar loss of freshness as a beer that spent three months at 70F. Again, remember that loss of freshness can be good or bad, depending on the beer style, and the goal for the aging of the beer. The recommended temperature for long-term aging of beer is 55F. I think of that as an upper limit. Below that temperature, the reactions will still occur, albeit at a slower rate. Above 55F, the reactions will occur at such a speed that the beer ages perhaps inappropriately. A useful generalization when thinking about this concept comes from Swedish chemist Svante Arrhenius, who determined that the reaction rate doubles for most common chemical reactions for every 10C increase in temperature, for low-energy barrier reactions. High-energy barrier reactions occur 8 times faster. Basically, some aging reactions in your beer are occurring twice as fast at 73F, compared to 55F, and some are occurring 448 times faster. Of course, many other variables are at play here: the rate of reactions does not always increase proportional to the temperature increase. Now, think of imported beer that has been in a shipping container for weeks, or your imperial stout that hits 80F in the summers. A few weeks, or even a few days, at these high temperatures could make a one month old beer taste like it has been aged for a year, which is rarely a good thing. Moreover, increased temperature promotes the growth of beer spoilage organisms, if present.
Additionally, high temperatures may encourage chemical reactions that are absent, or rare, at normal cellar temperatures, resulting in an inappropriately-aged flavor profile, as opposed to an expected aged flavor profile. The impact of these changes depend on your ability to taste them, the flavor thresholds of the compounds, and the overall character of the beer. A forced-aging test is an experiment that is really worth trying out at home (which I mentioned in one of the other installments): take two bottles of the same beer, a homebrew or commercial brew, put one in the fridge, and put the other in a warm area. I put mine in the oven on the lowest setting (mine goes down to 170F) for a few hours. After the beer is stored warm for a period of time, open both bottles and compare the flavor differences. You may be able to force-age your beer at 90F, instead of keeping it at 55F for a length of time. But, be advised: the window at which the flavor will be balanced and appropriate will be very small. Remember the rate of reactions, as mentioned previously. At excessively high temperatures, some aging, oxidative, and/or staling reactions are occurring staggeringly fast, whereas these reactions may not occur at all when the beer is aged at an appropriate cellar temperature.
How does an increase in temperature influence the flavor? In general, the same set, or type, of reactions will occur over a shorter time frame. Recall the Dalgliesh chart from part 1 that describes the general change in the flavor profile of the beer over time. You must compress the graph, then extrapolate (or extend) the lines. However, the dynamics of the changes are influenced by the style of the beer, among other things. For example, the sweet taste described in the chart is not a single reaction, but a large group of reactions that can occur, both, simultaneously, and sequentially. That bottle that has been sitting on top of your fridge (a notoriously bad place to cellar beer) has been subjected to heat, not to mention light and vibrations. Imagine, then, how this environment will influence the chart.
*Keep in mind that this is a generalization. The dynamics of the changes occurring in your beer may not always simulate this chart.
But what about temperature cycling during storage? Does cycling your beer between warm and cool do more damage than just warm temperatures? This topic has been debated pretty extensively. The Master Brewers Association of America says that deterioration of beer flavor only occurs at high temperatures. The high temperature drives the reactions, but the low temperature slows the reactions. The time spent warm causes the damage to the beer, and not the act of cycling through temperatures. The greatest impact of temperature cycling is in the clarity of your beer: cycling can turn temporary chill haze into permanent haze.
This article comes with the usual caveat: semantics. Descriptors such as flavor deterioration, damage, and, aged can not only be subjective, but relative to the goal of your beer as well. The flavors in a Russian Imperial Stout may technically have deteriorated during long-term aging, but those deteriorated flavors are what give an aged RIS its characteristic profile. Heat is punishing to your beer. Increased temperatures drive oxidation reactions and encourage the growth of spoilage organisms. Once your fermentation is complete, freshness and stability of your beer is best preserved by storing it as cold as possible, but any warmer than that, and you’re likely looking at some unexpected off-flavors.